DeflaGyn
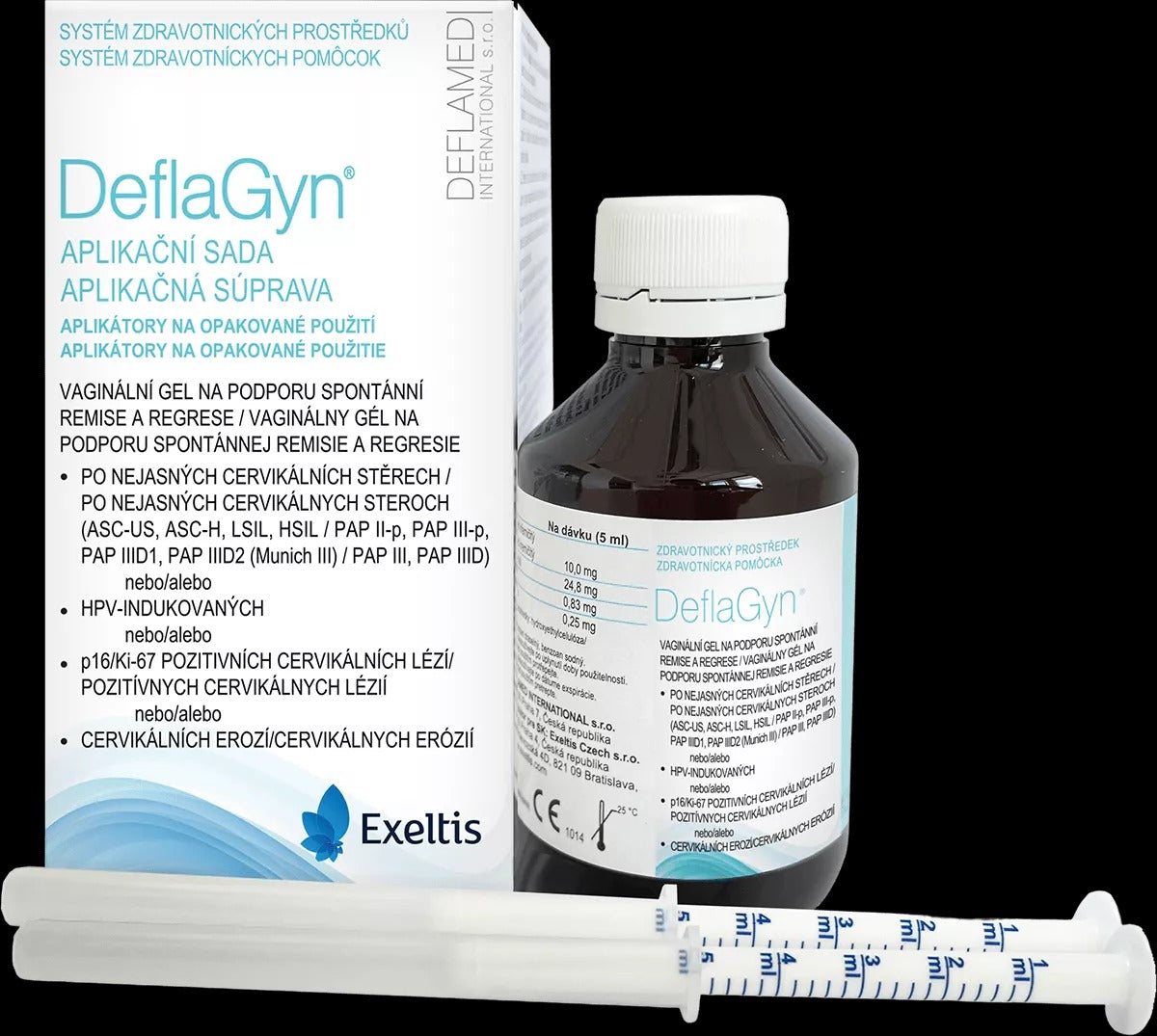
DeflaGyn Application Kit Vaginal Gel 150 ml + 2 Applicators
DeflaGyn Application Kit Vaginal Gel 150 ml + 2 Applicators
Couldn't load pickup availability
DeflaGyn Application kit vaginal gel containing silica helps to quickly normalize (remission) findings on the cervix. It binds pathogens (agents of changes) and prevents their further spread. With a strong antioxidant effect, it protects the surrounding healthy cells from possible further infection. Deflagyn is used when an unclear cervical smear is found (ASC-US or LSIL, HSIL). DeflaGyn® should be used for 3 months (3×28 days) while waiting for a control smear from the cervix.
Usage instructions:
Using an applicator, apply 5 ml of gel deep into the vagina.
Use Deflagyn for 3x28 days. After 28 days of treatment, discontinue the use of the gel for 3 days. Then continue treatment for another 28 days and stop for another 3 days. Then proceed to the third course of treatment.
Do not use vaginal gel during period (about 3-5 days per cycle). It is not necessary to interrupt the treatment for another 3 days, since menstruation is already counted as a break in treatment.
Shake the bottle well before use! After use, part of the applied gel can drain. This gel may appear slightly reddish and may stain your underwear. After washing the laundry, the coloring disappears. You can also use a sanitary napkin.
Ingredients:
Highly dispersed micronised silica 10 mg, citric acid 24.8 mg, sodium selenite 0.83 mg, selenium equivalent 0.25 mg at a dose of 5 ml. Supporting means: hydroxyethylcellulose, water; preservatives: potassium sorbate, sodium benzoate.
Notification
Do not use with known hypersensitivity to any of the ingredients of the product. DeflaGyn® vaginal gel should only be used after consulting a doctor, especially during pregnancy.
MEDICAL DEVICE, READ CAREFULLY THE INSTRUCTIONS FOR USE.
Share